Curcuvail- Turmeric & Curcumin Extract
Introducing inventive Curcumin formula for advanced Bioavailability!!
Curcuvail by K. Patel Phyto Extractions Pvt. Ltd
Turmeric & Curcumin Manufacturers & Suppliers India is a unique innovative curcumin, specially designed using dispersion technology.
What is Turmeric ?
Turmeric (Curcuma longa, an Indian spice), a herbaceous plant of ginger family, Zingiberaceae, the roots of which has traditionally been used in cooking. Turmeric is a plant that has a very long history of traditional use, dating back nearly 5000 years where Indian Ayurvedic science recognised it. In Southeast Asia, turmeric is used not only as a principal spice but also as a component in religious ceremonies. Because of its brilliant yellow color, turmeric is also known as “Indian saffron.” Of late, modern nutritional and medicine science has begun to recognise its value and importance. This is indicated by the presence of over 6000+ papers and publications in just last three decades. India grows world’s 85% Turmeric roots of high quality. The bioactive constituents of Turmeric are curcumin, demethoxycurcumin, bis-demethoxycurcumin and more than 30 essential oils.
In Southeast Asia, turmeric is used not only as a principal spice but also as a component in religious ceremonies. Because of its brilliant yellow color, turmeric is also known as “Indian saffron.” Of late, modern nutritional and traditional science has begun to recognise its value and importance. This is indicated by the presence of over 6000+ papers and publications in just last three decades. India grows world’s 85% Turmeric roots of high quality.
What is Curcumin ?
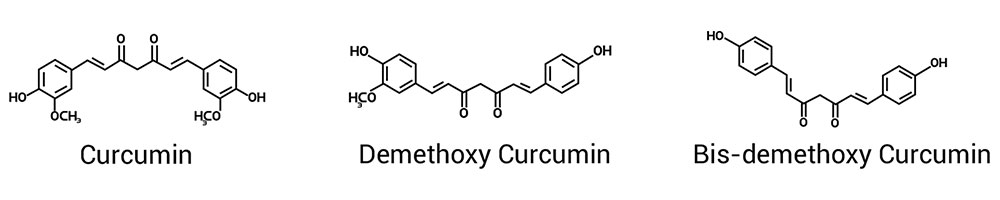
Commercially Curcuminoids are known as Curcumin which contains three polyphenols namely, curcumin, demethoxycurcumin and bis-demethoxycurcumin. Curcumin is a powerful and safe natural remedy with proven health benefits
Benefits
Various studies have shown that Curcumin helps in
Joint & muscles health,
Supports cognitive function,
Helps with Normal Joint Function,
Supports the body’s natural detoxification process,
Supports Healthy Cardiovascular Function,
and Promotes healthy mood balance.
What is Curcumin ?
Commercially Curcuminoids are known as Curcumin which contains three polyphenols namely, curcumin, demethoxycurcumin and bis-demethoxycurcumin. Curcumin has diverse therapeutic / pharmacological actions with well documented pharmacokinetics, efficacy & safety.

It has interactions with numerous targets, inhibiting activation of transcription factors, down regulation of the activity of multiple kinases, inhibits expression of growth and metastasis promoting genes, cell invasion and adhesion, regulate activities of several enzymes that mediate tumor growth and so on. Curcumin regulates the expression of inflammatory enzymes, cytokines, adhesion molecules, and cell survival proteins.
Benefits
Various studies have shown that Curcumin helps in
Joint & muscles health,
Boosts cognitive function,
Helps with Normal Joint Function,
Boosts detoxification,
Promotes Healthy Cardiovascular Function,
and Promotes healthy mood balance.
What is CurcuVail®
CurcuVail® is a unique innovative Curcumin that is specially designed using dispersion technology. It has evolved and extended traditional goodness of Curcumin by enhancing its bioavailability.
Why CurcuVail®
Conventional Curcumin owing to its poor bioavailability leads to lower efficacy in any application made from it.
A highly bio-available and better water dispersible substance helps quicker absorption in human body. The enhanced bioavailable CurcuVail® helps design and configures applications that increase its absorption into human body leading to faster end effect.
Salient Features of CurcuVail®
- No Residual solvent.
- Zero Pesticides residue
- No Odor and Taste of turmeric
- Completely water dispersible
- Easy to clean the equipment after process
- No bioavailability enhancer required
- Non GMO
- Self affirmed GRAS
- User friendly for all applications like Beverage, Food, Confectionaries, Nutraceuticals, Premix, Cosmetics etc.
Applications

Use of CurcuVail® helps to reduce dosage as the higher bioavailability increases efficacy of formulation. This makes the formulation smarter and effective.

Higher dispersion attribute will help beverage absorb CurcuVail® more homogeneously into water. This will give beverage a better color and taste that does not interfere with original product flavor.

CurcuVail® acts as a non-synthetic colorant with its nutritional values intact. It does not interfere with the original taste of the food product.

Confectionery and baked products are exposed to a higher temperature in their cooking process. CurcuVail® remains stable with higher heat exposure and retains the product’s taste properties.

CurcuVail® as an ingredient perfectly blends with any medium as nutrient and absorbs into the water seamlessly. This gives a premix an ideal palatable blend and taste.

Use of CurcuVail® will help your end product for appropriate end effective brand communication supported by the above values. There are clinical studies to support the same for authenticity and accuracy.
Our CurcuVail® is backed by Human Clinical studies
Bioavailability
Joint Health
Supports Immune Health
Liver Health
Clinical Trial
Objective: To compare bioavailability of CurcuVail® with Curcumin 95%.
Dose: 500mg
Number of subjects: 24 (two groups of 12 each)
An open label, balanced, randomized, two-treatment, two-period, two-sequence, single dose, crossover, relative, bioavailability study of Turmeric Extract (Curcumin) 500mg capsule with Turmeric bio-available extract 500mg capsule of CurcuVail®, in healthy 24 adults human subjects under fasting conditions.
Results: Mean Plasma concentration (ng/ml) Vs Time (h) of Curcumin.
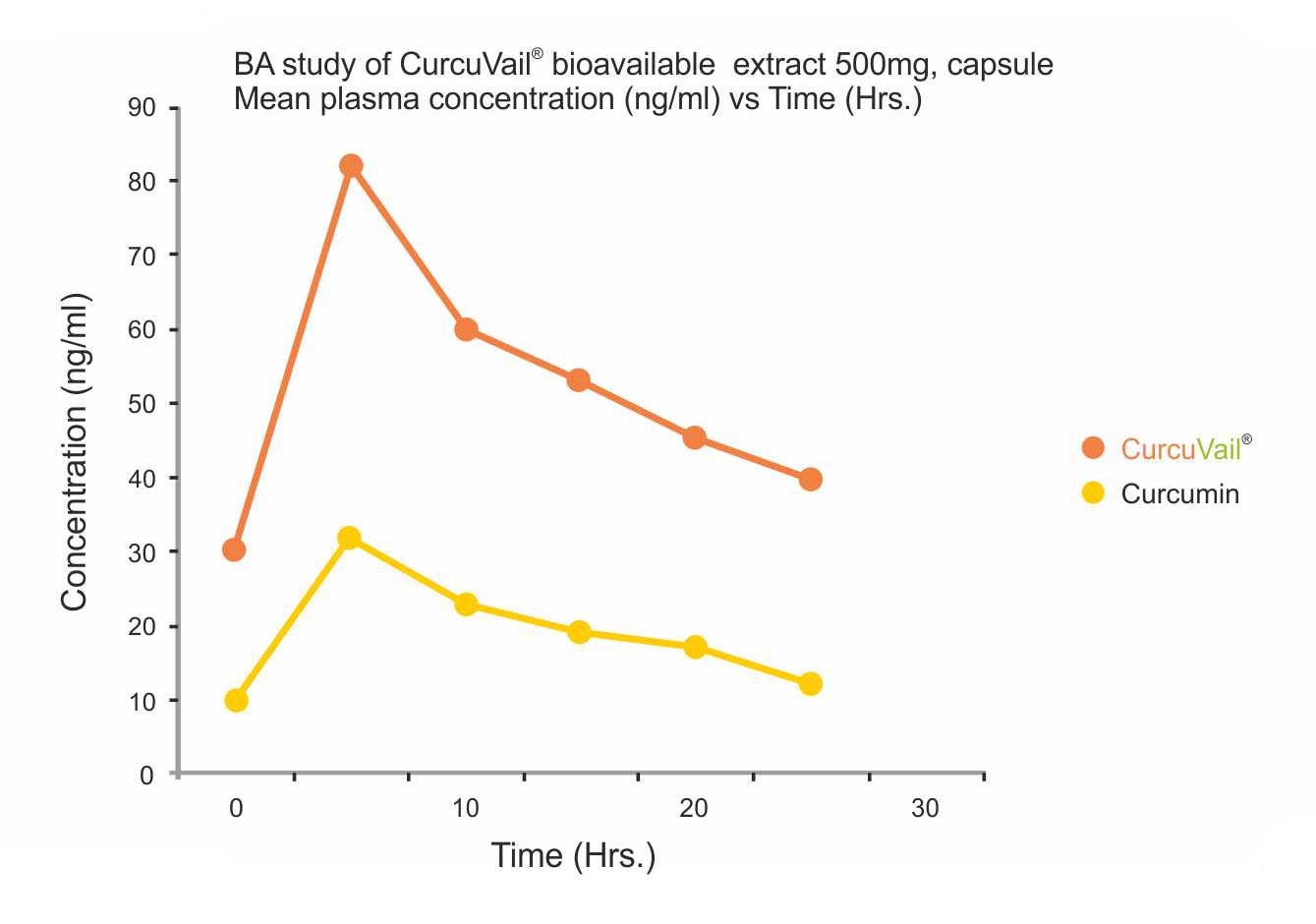
Cmax: CurcuVail® showed a significantly higher concentration of Curcumin in blood plasma compared to Curcumin 95%.
The mean concentration of Curcumin was found to be 82ng/ml for CurcuVail® compared to 36ng/ml for Curcumin 95% at Tmax of around 5 hours.
Conclusion: CurcuVail® has substantially significant absorption of Curcumin in the blood as compared to Curcumin 95%. This study shows that CurcuVail® is more than 3X times absorbed in the blood or 9X more bioavailable when compared with Curcumin at same concentration.
“A Prospective, Interventional, Randomized, Parallel, Double blind, Placebo Controlled Clinical Study to evaluate the Efficacy & Safety of Curcumin capsule (CurcuVail®) in Patients with cough due to Chronic Bronchitis.”
Objective: To assess the efficacy of Curcumin capsule (CurcuVail®) in Patients with cough due to Chronic Bronchitis.
Dose: 500mg / day
Duration: 10 weeks
Number of subjects: 40
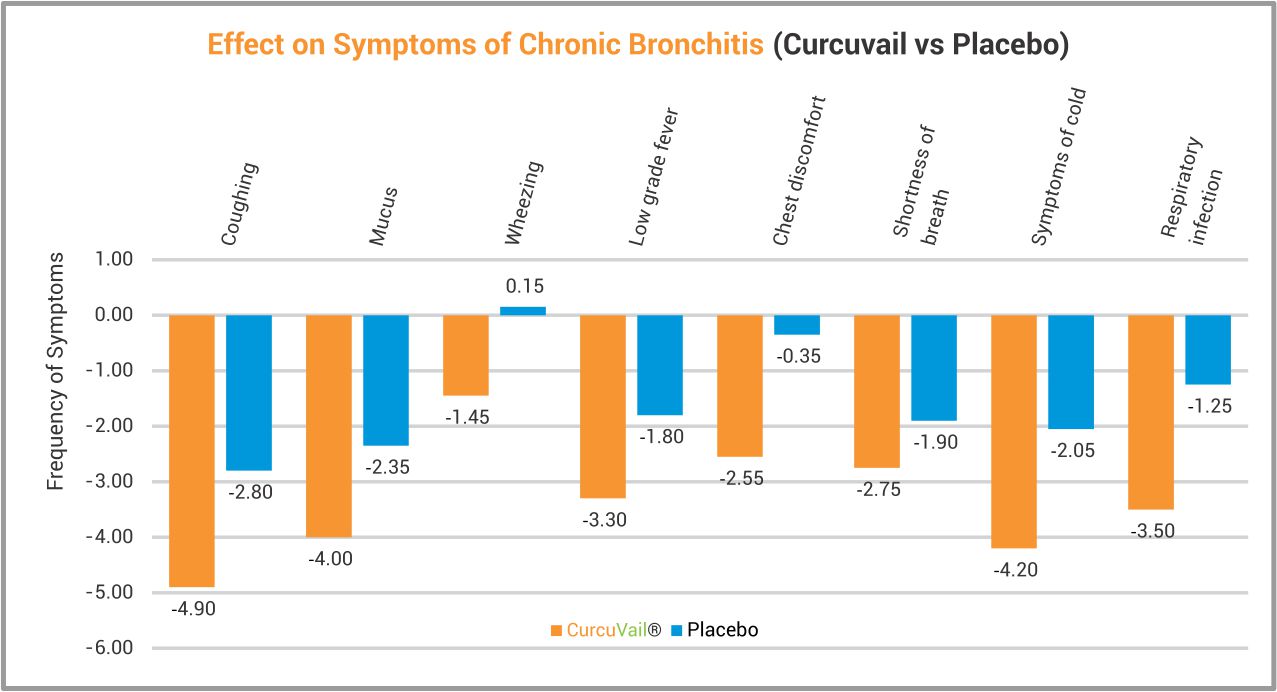
At End of Treatment, CurcuVail® statistically showed significant improvement in symptoms of chronic bronchitis such as Coughing, Mucus secretion, Wheezing in lungs, Low grade fever, Chest discomfort, Shortness of breath, Cold and Recurrence of respiratory infection.
Chest X-ray analysis revealed significant improvement in mucus clearance of bronchi. Immunological parameters such as total IgG, IgM and IgE level were also markedly improved in comparison to Placebo group.
Conclusion: The study concludes that CurcuVail® (Special Curcumin) owing to its anti-inflammatory and immuno-modulatory effect, was not only safe but also significantly efficacious in treatment of all major symptoms of Chronic bronchitis. This study has also proven potential of CurcuVail® as marked immunity enhancer.
A Prospective, Interventional, Randomized, Parallel, Double blind, Placebo Controlled Clinical Study to evaluate the Efficacy & Safety of Curcumin capsule CurcuVail® in Patients with Chronic joint pain (Rheumatoid Arthritis).
Objective: To show the efficacy of CurcuVail® in Patients with Chronic joint pain (Rheumatoid Arthritis).
Dose: 500mg / day
Duration: 12 weeks
Number of subjects: 30
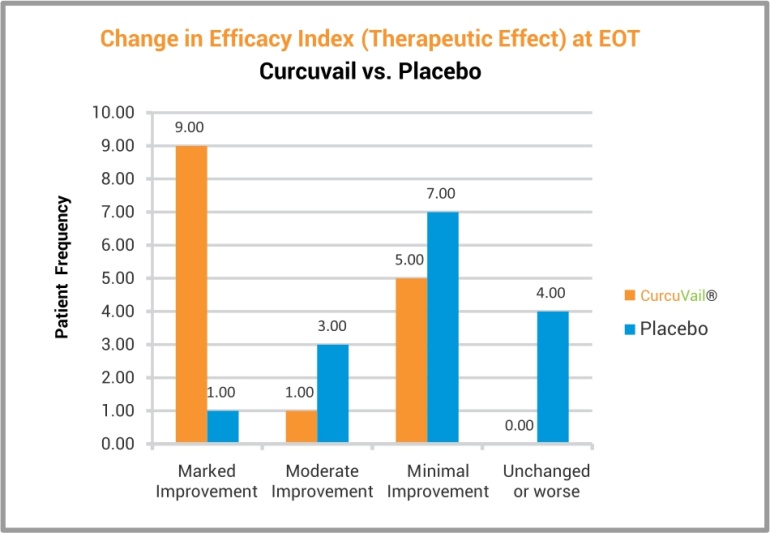
At the end of the study, statistical analysis of efficacy using CGI (Clinical Global Impression) scale and Global improvement Score, suggested that the patient group treated with CurcuVail® showed marked improvement in overall therapeutic effect as compared to Placebo group.
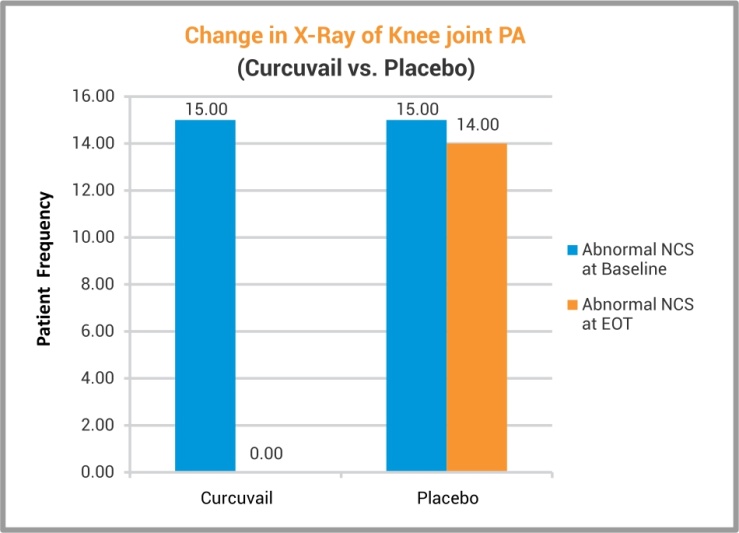
X-ray analysis of Knee joint PA has revealed that the group treated with CurcuVail® exhibited remission of inflammatory anomalies of joint in all patients which proved anti-inflammatory potential of CurcuVail® as compared to Placebo group.
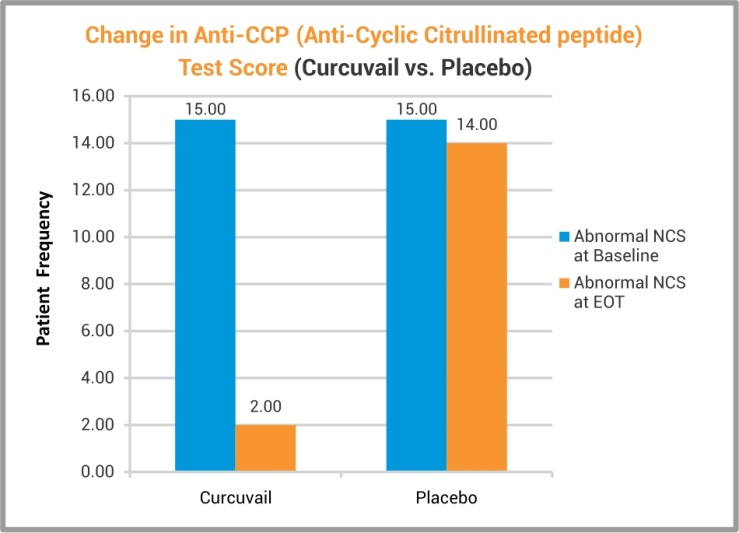
Anti-CCP (anti-cyclic citrullinated peptide) analysis in blood showed significant correction in this RA specific marker in the patients who received CurcuVail® treatment. This result indicated certain efficacy of CurcuVail® on immunity markers and so as in reversal of arthritic changes.
Conclusion: The study concludes that, TEST (CURCUMIN) due to its anti-inflammatory and immunomodulatory effect it is more efficacious and safer in comparison to PLACEBO (B) in treatment of chronic joint pain due to Rheumatoid Arthritis.
A prospective, randomized, double blind, placebo controlled, parallel group study to evaluate the safety and efficacy of CurcuVail® of K Patel Phyto Extractions Pvt. Ltd. in patients with Non-alcoholic Fatty Liver Disease (NAFLD)
Objective: Evaluation of the safety and efficacy of CurcuVail® in patients with Non-alcoholic fatty liver disease (NAFLD)
Dose: 250 mg capsule
Number of subjects: 30 (two groups of 15 each)
Duration: 2 months
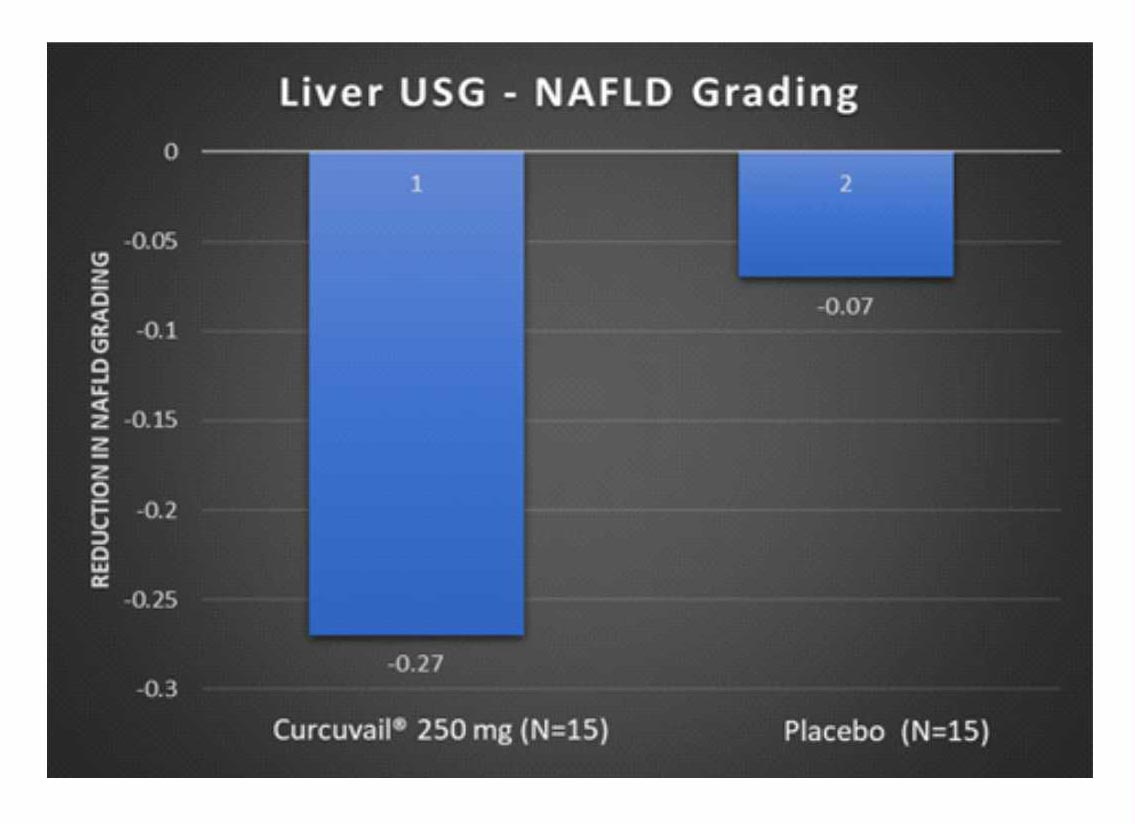
Change from baseline in NAFLD grading
NAFLD patients are mostly asymptomatic; however, asymptomatic liver enzyme elevation gives a clue to the diagnosis. The necroinflammatory grades of NAFLD as Grade 1 (mild), Grade 2 (moderate), and Grade 3 (severe) based on the degree of hepatocellular steatosis, ballooning and disarray, and inflammation (intralobular and portal).2
Conclusion: – 4 patients out of 11 patient of CurcuVail® treatment group were found with decrease in fatty liver grading from grade 2 to grade 1 as compared to 1 out of 14 of Placebo group.
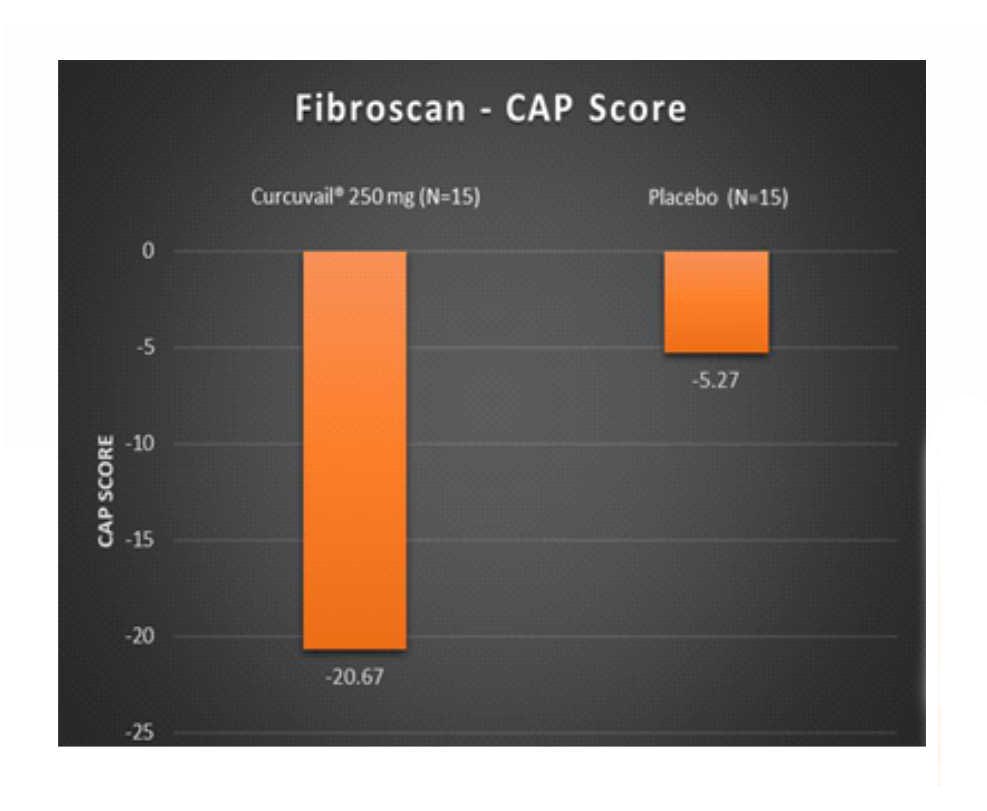
Change in Fibroscan parameters at the end of treatment.
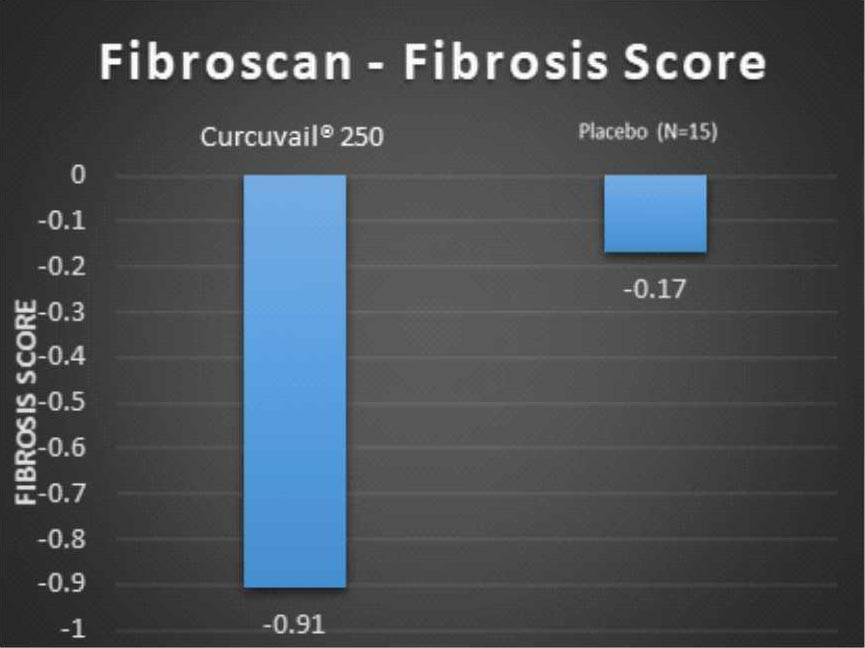
Fibrosis score measures the level of scarring to the liver. The greater the fibrosis score, more severe the liver damage.
CAP score is a measurement of fatty change in the liver. It is used to assess steatosis (fatty changes) grade in liver tissue.
Conclusion: – The CurcuVail® treatment showed (statistically highly significant) almost 5.4 times more decrease in fibrosis score and almost 3.9 times more decrease in CAP score when compared to placebo treatment.
CONCLUSION
- Test drug CurcuVail® was found to be efficacious in reducing NAFLD grading by Liver USG. APRI score, Fibrosis score, CAP score, Total cholesterol, Serum AST and Serum ALT at treatment duration of 2 months.
- CurcuVail® drug has shown statistically significant impact on Liver inflammatory markers such as AST, ALT and APRI Score confirming its efficacy on relieving liver inflammation.
About Us
K. Patel Phyto Extractions Pvt Ltd., is a member of K. Patel Group of companies. It is NSF-GMP certified manufacturer of Botanical Extracts and Phytochemicals from India. K. Patel exports to more than 30 countries and also has a strong presence in the Indian market. K. Patel presently offers a portfolio over 40 products.
Manufacturing Facility
Spread across 32 acres of land K. Patel Phyto Extractions facility has been audited by US FDA and NSF-GMP. The ultra modern top of the line machineries installed are capable of charging 5000MT of raw material annually.
Well equipped Lab with modern instruments, testing devices and qualified personnel for careful identification of analytical methods. Additionally K. Patel offers pesticides testing as per USP and Heavy Metals as per ICP-MS in third party lab. Improvement is a continuous activity so K. Patel has an independent R&D and Pilot plant for developmental activities. At K Patel we assure you of Quality, Consistency, Assured delivery and Fair price products.
Sourcing
K. Patel works on 360ºSCM to ensure that it offers traceability till farms for our botanical extracts. It has carefully identified the regions for its focused herbs. It educates the farmers to follow GAP (Good Agricultural Practices) and offers guidance to maximize the yields. K. Patel also promotes contractual farming for organic herbs.
Manufacturing Facility
Spread across 32 acres of land K. Patel Phyto Extractions facility has been audited by US FDA and NSF-GMP. The ultra modern top of the line machineries installed are capable of charging 5000MT of raw material annually.
Well equipped Lab with modern instruments, testing devices and qualified personnel for careful identification of analytical methods. Additionally K. Patel offers pesticides testing as per USP and Heavy Metals as per ICP-MS in third party lab. Improvement is a continuous activity so K. Patel has an independent R&D and Pilot plant for developmental activities. At K Patel we assure you of Quality, Consistency, Assured delivery and Fair price products.
We have current accreditations from :


Analytical Specification Of CurcuVail®
| PLANT PART USED |
| Rhizome |
| COMMON NAME |
| Turmeric, Haldi |
| DOC.NO & REV.NO. |
| QC/CS/1002 & 05 |
| SUPERCEDES |
| QC/CS/1002 & 08.02.2019 |
| HERB RATIO |
| 21 – 32 :1 |
| COUNTRY OF ORIGIN |
| INDIA |
| STERILIZATION |
| Heat |
| GMO & IRRADIATION STATUS |
| Non GMO & Non Irradiated |
| Scientific name of ingredient is Curcuma longa | ||||
|---|---|---|---|---|
| PARAMETERS
Description Water Dispersion Identification Moisture content (By KF) pH |
UM
– – – % w/w – |
SPECIFICATION
Yellow Orange coloured powder having characteristic odour. Dispersable in water Positive for Curcuminoids Not more than 8.00 Between 4.0 – 8.0 |
METHOD
Organoleptic In-house method QC/STP/HB/28 QC/STP/GAM/01 QC/STP/GAM/01 |
|
| Heavy metals
Lead Arsenic Cadmium Mercury |
ppm
ppm ppm ppm |
Not more than 5.0
Not more than 2.0 Not more than 0.3 Not more than 0.1 |
ICP – MS
ICP – MS ICP – MS ICP – MS |
|
| Assay:
Curcuminoids content (by HPLC) |
% w/w | Not less than 35.00 | QC/STP/HB/28 | |
| Microbiological analysis:
Total plate count Yeast & mold count |
cfu/gm
cfu/gm |
Not more than 3000
Not more than 100 |
QC/MOA/MIC/05
QC/MOA/MIC/05 |
|
| Pathogens:
Escherichia coli Salmonella Pseudomonas aeruginosa Staphylococcus aureus Carrier Excipients |
–
– – – – |
Absent / 10 gm
Absent / 25 gm Absent Absent / 10 gm Maltodextrin |
QC/MOA/MIC/05
QC/MOA/MIC/05 QC/MOA/MIC/05 QC/MOA/MIC/05 |
|
Grade: Food and Pharma
Purpose: Food and Food Supplement
Shelf life: 2 years from the month of manufacture
Standard Packaging details :
Net 20 / 25 kg product in double lined polybag, sealed with tie lock sequentially. The sealed bags are kept in
HDPE drums with silica gel placed on the bags and covered by HDPE lid, clamped and locked with Plastic tamper
seal lock .
Storage: Store in dry & tight containers
Remarks: Since the product is derived from natural origin there is possibility of marginal dissimilarity in colour
o wing to variations in ecological, climatic and source of raw material.
Published Papers
Our Branded Ingredients
(Clinically Proven)